소아 흡인의 위험인자: 비디오투시조영 삼킴검사 연구
초록
배경 및 목적
본 연구는 흡인을 보이는 삼킴장애 소아환자와 흡인을 보이지 않는 삼킴장애 소아환자의 비디오투시조영 삼킴검사(Videofluoroscpic Swallowing Study)를 분석하여 두 집단을 효과적으로 구별할 수 있는 흡인 위험인자를 찾고자 하였다.
방법
삼킴장애로 VFSS가 의뢰된 42명의 환아(연령: 1-3세 11개월)가 본 연구에 참여하였으며 흡인 유무에 따라 두 집단으로 나뉘었다. 각 집단의 VFSS 결과에서 7가지 삼킴시간 측정치를 분석하였고 두 집단을 구별하는 데 유용한 측정치를 찾기 위해 다수준모형 분석을 실시하였다.
결과
지연인두삼킴, 인두통과시간, 후두폐쇄유발시간이 두 집단을 유의미하게 구별하는 것으로 나타났다. 단일 측정치로는 지연인두삼킴이 두 집단을 식별하는 데 가장 유용한 측정치로 나타났으며 후두폐쇄유발시간이 뒤를 따랐다. 지연인두삼킴+인두통과시간 모델이 집단 소속을 예측하는 데 가장 효과적이었으며, 인두통과시간+후두폐쇄유발시간 모델이 두 번째로 효과적이었다.
논의 및 결론
본 연구결과, 삼킴장애를 보이는 소아 환자의 VFSS에서 음식덩이가 인두에 오래 남아있으면서 인두삼킴 시작이 지연되는 경우와 음식덩이가 인두에 오래 위치하고 있을 때 기도 닫힘이 지연되어 일어나는 경우 흡인 위험이 높아지는 것으로 나타났다. 본 연구의 결과는 임상가가 VFSS를 실시할 때 필수적으로 고려해야 하는 흡인의 위험인자에 대해 제시하고 있으며 추후 병리학적 흡인 원인에 따른 적절한 치료를 시행하는 데 도움이 될 것이다.
Keywords: 소아삼킴장애, 흡인, 비디오투시조영 삼킴검사
Abstract
Objectives
This study examined to identify effective temporal measures to distinguish between aspirators and non-aspirators in young children with dysphagia.
Methods
Fortytwo children aged from 1 year to 3 years 11 months were included in this study. All children were referred to Videofluoroscopic Swallowing Study (VFSS) at a tertiary hospital in South Korea. The children were divided into two groups depending on the presence of aspiration: aspirators (N=16) and non-aspirators (N=26). Seven temporal measures were measured to identify swallowing physiologies by using frame-by-frame analysis. A multilevel model with binomial distribution was used to examine which temporal measures better predict group membership.
Results
Delayed pharyngeal swallow (DPS), pharyngeal transit time (PTT), and initiation of laryngeal closure (ILC) were significant factors in distinguishing the aspirators from the non-aspirators. DPS was the most useful temporal measure to distinguish between the groups. ILC placed second, followed by PTT. A model including DPS and PTT was the best model to predict the group membership. A model with PTT and ILC ranked second.
Conclusion
The findings indicated that an aspiration risk appeared to increase when the bolus stayed longer in the pharynx and initiation of pharyngeal swallow was delayed. Also, the bolus remained in the pharynx for an abnormally longer time while the airway was open in the aspirators. This study suggests that clinicians should not only recognize pathophysiological factors that may increase the aspiration risk but also determine which abnormal physiology is a main factor of aspiration during VFSS.
Keywords: Pediatric dysphagia, Aspiration, Temporal measures, Videofluoroscopic swallowing study
The incidence of dysphagia (i.e., swallowing disorders) has increased alongside an improvement in the survival rate of children with a history of prematurity and life-threatening medical disorders ( Arvedson, 2008; Lefton-Grief, 2008). Multiple factors contribute to dysphagia in pediatric populations, including a history of prematurity, neurological disorders, respiratory disease, congenital cardiac disease, craniofacial anomalies, airway malformations, and gastrointestinal disease ( Dodrill & Gosa, 2015; Friedman & Frazier, 2000; Lefton-Greif & Arvedson, 2008; Newman, Keckley, Petersen, & Hamner, 2001; Roden & Altman, 2013). Previous studies reported that children having swallowing problems in the oral stage used more energy expenditure during oral intake, preventing them from maintaining and gaining weight ( Erasmus, van Hulst, Rotteveel, Willemsen, & Jongerius, 2012; Lustre, Freire, & Silverio, 2013). Also, dysphagia in children with neurological disorders was associated with malnutrition and delayed development ( Han, Bang, Chung, Shin, & Jeon, 2001). Among swallowing disorders in oropharyngeal swallowing, aspiration is considered to be a major concern because chronic or recurrent aspiration can lead to pulmonary problems, including recurrent wheezing, recurrent pneumonia, and bronchiectasis in pediatric populations ( Tutor & Gosa, 2012; Weir et al., 2007). Infants and young children are more likely to have silent aspiration than adults due to an absent or ineffective cough reflex ( Velayutham et al., 2018; Weir et al., 2007). Thus, it is important to identify pathophysiological factors that can distinguish children with a high risk of aspiration from those without them. Videofluoroscopic Swallowing Study (VFSS) is considered the primary instrument to examine swallowing function. It shows how food or liquid passes from the mouth through the pharynx. Specifically, VFSS provides clinicians with information about the timing and underlying pathophysiological factors of aspiration ( Logemann, 1998). Temporal measurements from VFSS have been used to understand swallowing physiology and pathophysiology in each swallowing stage by providing objective values in adult populations ( Logemann, Pauloski, Rademaker, & Kahrilas, 2002; Logemann et al., 2000). However, it remains unknown whether temporal measurements that were used in adult populations can be applied to pediatric populations to explain swallowing pathophysiology throughout the entire swallowing process in children with dysphagia. Specifically, it is necessary to identify valid and reliable temporal measures distinguishing swallows that are indicative of aspiration risk.
Han, Kim, Yi, and Oh (2024) examined the temporal measures significantly showing the difference between aspirators and nonaspirators in pediatric populations. In addition to the previous study, this study investigated which temporal measures are more effective in distinguishing between aspirators and non-aspirators. The specific research aims were to examine which temporal measure better predicts group membership (i.e., aspirators vs. non-aspirators) and to build models for predicting group membership. The findings of the current study will help clinicians perform swallowing management strategies tailored to the pathophysiological factors to reduce the risk of aspiration and its medical complications in pediatric populations.
METHODS
This study was a retrospective study that reviewed VFSS data and medical charts. Ethics approval for the study was obtained from the institutional review board of Ohio University (IRB No: 19-E-215) and Seoul National University Hospital (IRB No: H-1906-144-1043).
Participants
The participants included in the current study were drawn from the same pool as those in the previous study ( Han et al., 2024). Forty-two children aged from 1 year to 3 years 11 months were included and divided into two groups according to the presence of aspiration. A child classified as an aspirator had experienced aspiration in at least one of the four trials. The aspirators comprised sixteen children with an average age of 21.2 months (SD=8.3 months, range=12-42 months). On the other hand, a child who had not exhibited aspiration in any bolus, meaning no instances of aspiration in all four trials, was assigned to the non-aspirator group. Twentysix children with an average age of 26.3 months (SD=8.4 months, range=14-46 months) belonged to the non-aspirators. This study focused on the swallowing function in young children or toddlers. In general, toddlers are considered to be 1 to 3 years of age ( American Academy of Pediatrics, n.d.). For the current study, the age range was extended to include children who range in age from 3 years 1 month to 3 years 11 months because it was assumed that the swallowing function of the children was less developed as compared to children in the same chronological age due to their etiologies (e.g., prematurity, congenital abnormalities – syndrome, neurological disorders, etc.). The etiologies in the children included neurological disorders (e.g., hypoxic-ischemic encephalopathy, cerebral palsy, encephalitis, brain tumor, neuromuscular disease, seizure, spinal cord diseases) (84%), congenital abnormalities (e.g., Edward syndrome, Down syndrome) (9%), cardiopulmonary disorders (e.g., primary pulmonary hypertension, heart transplant status) (5%), and nonspecific (2%). The demographics and clinical information for each group are presented in Table 1.
Videofluoroscopic Swallowing Study (VFSS)
VFSS was conducted at the swallowing clinic of the Department of Pediatric Rehabilitation Medicine at Seoul National University Hospital. Swallowing assessments were carried out using either digital videofluoroscopy or C-arm technology at 30 frames per second. Each child was positioned in an upright seated posture, using either a tumble-form chair or a bush chair with back/lumbar support if additional support was needed for the child’s head and body positioning during VFSS. The lateral view of VFSS was obtained for all the children. A child swallowed a thin liquid and puree, both fed by a spoon. The thin liquid was a mixture of milk formula or water and barium sulfate powder (35%w/v), conforming to the zero level of the International Dysphagia Diet Standardisation Initiative (IDDSI). The puree, composed of Yoplait and barium sulfate, adhered to level four of IDDSI. Each consistency type was ingested twice, resulting in a total of four swallowing trials per participant. The bolus volume for each trial was 2 mL.
Procedures for Temporal Measurements
The presence of aspiration for all swallows and seven temporal measures were analyzed by using frame-by-frame analysis with Adobe Premiere Pro CS5.5. The valleculae were used as a reference point for triggering the pharyngeal swallow in young children as reported in previous research ( Newman, Cleveland, Blickman, Hillman, & Jaramillo, 1991; Weckmueller, Easterling, & Arvedson, 2011). The eight anatomical and physiological references were used for the temporal measurements; (1) initiation of tongue elevation, (2) bolus head reaching the valleculae, (3) bolus tail passing the UES, (4) initiation of laryngeal elevation, (5) initiation contact of arytenoids and epiglottis, (6) final contact of arytenoids and epiglottis, (7) initiation of opening of the UES, and (8) closure of the UES ( Figure 1). The seven temporal measures were as follows.
1) Oral Transit Time (OTT): the time from tongue tip elevation to the arrival of the bolus head at the valleculae (Power et al., 2009). 2) Delayed Pharyngeal Swallow (DPS): the time of the initiation of laryngeal elevation in relation to the frame at which the bolus head reaches the valleculae (Perlman, Booth, & Grayhack, 1994). 3) Pharyngeal Transit Time (PTT): the time from the arrival of the bolus head at the valleculae to the bolus tail passing the upper esophageal sphincter (UES) (Power et al., 2009). 4) Initiation of Laryngeal Closure (ILC): the duration between the bolus head passing the valleculae and the first contact of arytenoids to epiglottis (Park, Kim, Ko, & McCullough, 2010). 5) Duration to achieve Laryngeal Closure (D to LC): the time from the initiation of laryngeal elevation until the first contact of the arytenoids and the epiglottis (Kendall & Leonard, 2001). 6) Laryngeal Closure Duration (LCD): the time from the initiation of laryngeal closure until the final contact of arytenoids and epiglottis (Power et al., 2009). 7) Duration of UES Opening (DUESO): the time from the initiation of UES opening to the closure of the UES (Robbins, Levine, Maser, Rosenbek, & Kempster, 1993).
Statistical Analysis
Discriminant analysis was initially considered as a statistical tool to identify factors of aspiration. However, due to numerous dropped cases resulting from violations of assumptions required for discriminant analysis, a multilevel model with binomial distributions was used to examine which of the seven temporal measures better predicted group membership (i.e., aspirators vs. non-aspirators). Given the moderate to high correlations between these seven temporal measures, each of the seven temporal measures was separately entered into a model to predict group membership, following a procedure similar to forward selection in stepwise regression analysis. There was no built-in software to determine the best predictors in multilevel modeling. Therefore, each model was manually created using syntax with GENLINMIXED commands in SPSS. Since the multilevel model with binomial distribution was not built in SPSS, there was limited information accessible for factor selection compared to discriminant analysis. Hence, the F-value and p-value of each model were utilized to assess which temporal measures were more effective in predicting group membership. An F-test was used to determine the significance of each analysis, with the significant level set at p<.05. When more than one temporal measure had a pvalue less than .05 based on the fixed coefficients of each model, the temporal measures were modeled to determine which combinations of the temporal measures better predicted the group membership.
RESULTS
This study aimed to identify more effective temporal measures to distinguish between the aspirators and the non-aspirators. Table 2 provides the results of multilevel models using the temporal measures individually. Delayed pharyngeal swallow (DPS), pharyngeal transit time (PTT), and initiation of laryngeal closure (ILC) significantly distinguished the aspirators from the non-aspirators. The other measures did not significantly predict the group membership. Based on the p-value, DPS was the most useful temporal measure to distinguish between the aspirators and the nonaspirators (F (1, 98)=5.83, p=.018). Second, ILC was a useful temporal measure to predict the group membership (F (1, 98)=5.44, p=.022), followed by PTT (F (1, 98)=3.99, p=.049). Further analyses were carried out to investigate which combinations among DPS, PTT, and ILC better predict the group membership. A model including DPS and PTT was the best model to predict the group membership because it had the largest F-value and smallest p-value of the Type III test of fixed effects (F (2, 97)=3.65, p=.030). A model with PTT and ILC ranked second as a useful combination (F (2, 97)=3.53, p=.033). Entering all three temporal measures and a combination of DPS with ILC in a model did not significantly predict the group membership ( Table 3). In summary, DPS alone was the most effective temporal measure to distinguish between the aspirators and the non-aspirators. The combination of DPS with PTT was the best model for the prediction of the group membership.
Reliability
For reliability assessment, the primary investigator, who possessed 5 years of experience in swallowing temporal measurements and a research assistant with 1 year of relevant experience participated in the study. Intra-rater and inter-rater reliabilities were completed for the temporal measures using intraclass correlation coefficient (ICC). For intra-rater reliability, the first rater randomly selected 10% of swallows with aspiration and 10% of swallows without aspiration and analyzed the swallows. A significant correlation between the first and second ratings was found (r=.99 for OTT, r=.99 for DPS, r=.99 for PTT, r=.99 for ILC, r=.94 for D to LC, r=.99 for LCD, r=.97 for DUESO, p<.01). For inter-rater reliability, a second rater analyzed the same swallows. Significant correlations between the raters were found (r =.99 for OTT, r =.99 for DPS, r=.99 for PTT, r=.99 for ILC, r=.86 for D to LC, r=.98 for LCD, r=.98 for DUESO, p<.01).
DISCUSSION & CONCLUSION
Detailed values of the temporal measures in the two groups were presented in the previous research ( Han et al., 2024). Delayed pharyngeal swallow (DPS), pharyngeal transit time (PTT), and initiation of laryngeal closure (ILC) were identified to be valid to distinguish the aspirators from the non-aspirators. As a single variable, DPS emerged as the strongest temporal measure to predict the aspirator group, with ILC ranking second. This finding aligns with the results in the pediatric literature, where delayed initiation of pharyngeal swallowing and delayed initiation of laryngeal closure have been identified as the most common pathophysiology associated with aspiration ( Arvedson, Rogers, Buck, Smart, & Msall, 1994; Friedman & Frazier, 2000). In addition to the presence of DPS, the severity of delayed initiation of pharyngeal swallowing should be considered during VFSS because the presence of delayed pharyngeal swallowing does not imply that aspiration necessarily occurs. Perlman et al. (1994) reported that the occurrence of aspiration was associated with the severity of delayed initiation of pharyngeal swallow in adult stroke patients. Their stepwise logistic regression analysis revealed that a patient with severe delay was very likely to aspirate. However, a patient with mild delayed initiation of pharyngeal swallow was not more likely to aspirate than a patient without delay. In the current study, the aspirators having longer DPS represented a more severe delay and put a child at a higher risk of aspiration. Thus, clinicians should not only identify whether a child has delayed initiation of pharyngeal swallowing but also assess its severity. ILC was another variable that significantly distinguished the aspirators from the non-aspirators. In the present study, the pattern of prolonged ILC in the non-aspirators was interesting as compared to the pattern in the aspirators. For example, the value of ILC for three non-aspirators was prolonged but did not aspirate because their initiation of laryngeal closure occurred immediately when the bolus touched the aryepiglottic folds, which were inferior to the valleculae. The moment at which the bolus head reached the valleculae was used to calculate ILC in this study. Thus, the onset of laryngeal closure when the bolus was at the aryepiglottic folds below the valleculae may increase the value of ILC for the three children. On the other hand, the aspirators did not show immediate laryngeal closure even though the bolus touched and stayed on the aryepiglottic folds and pyriform sinus. It may be important to identify whether initiation of laryngeal closure occurs timely even though a trigger point of the pharyngeal swallowing is delayed. This will help clinicians to determine whether a child is at a high risk of aspiration. For the non-aspirators, it is assumed that a target movement (i.e., hyolaryngeal excursion to close the airway safely) may be achieved faster and more effectively to compensate for delayed initiation of the pharyngeal swallow despite the delayed onset of movement.
A prolonged PTT indicated that the bolus stayed longer in the pharynx due to weakness of pharyngeal muscles and was associated with an aspiration risk ( Henderson, Miles, Holgate, Peryman, & Allen, 2016; Leonard, Kendall, McKenzie, Gonçalves, & Walker, 2000; Yip, Leonard, & Belafsky, 2006). Although PTT itself was a useful temporal measure to predict the aspirator group, it seemed that PTT operated effectively in combination with the other measures. Steele and Cichero (2014) conducted a systematic review to investigate pathophysiological factors in association with aspiration. They concluded that an isolated pathophysiological factor may be insufficient to predict or explain aspiration, suggesting that a combination of multiple risk factors may result in high sensitivity and specificity to discriminate aspirators from non-aspirators ( Steele & Cichero, 2014). In the current study, further analysis was performed to identify which combinations of temporal measures (i.e., DPS, PTT, and ILC) predicted better the group membership. The combination of PTT with DPS and PTT with ILC strongly distinguished the aspirators from the non-aspirators, with only a .003 difference in their p-values. The findings indicated that an aspiration risk appeared to increase when bolus stayed longer in the pharynx and initiation of pharyngeal swallowing was delayed. Also, the bolus remained in the pharynx for an abnormally longer time while the airway was not closed ( Kahrilas, Lin, Rademaker, & Logemann, 1997; Park et al., 2010; Power et al., 2009). However, it should be considered that exhibiting one pathophysiological factor of aspiration neither necessarily accompanied the other factors nor resulted in aspiration ( Martin-Harris, Carson, Pinto, & Lefton-Greif, 2019). In other words, prolonged DPS was not always accompanied with longer PTT. Thus, clinicians should not only recognize pathophysiological factors that may increase the aspiration risk but also determine which abnormal physiology is the primary factor of aspiration when it is identified during VFSS. Identifying accurate physiological factors of aspiration will help clinicians perform appropriate management strategies to reduce the incidence of such factors. In the further analysis of the current study, contrary to expectations, the subset including DPS, PTT, and ILC together did not significantly predict group membership ( p=.065). One possible explanation for this may be a statistical phenomenon called multicollinearity. Namely, there were high correlations among the three measures in this study (DPS-PTT r=.945, p<.001; DPS-ILC r=.984, p<.001; PTT-ILC r=.954, p<.001). DPS, PTT, and ILC examined different swallowing physiological events. However, when the pharyngeal swallowing was triggered, these physiological events occurred either simultaneously or sequentially during the pharyngeal stage ( Logemann, 1998; Stevenson & Allaire, 1991). Therefore, the correlations among them may be inevitable. This explanation can also be applied to the combination of DPS with ILC that did not predict the group membership ( p=.058).
Limitations and Clinical Implications
There are several limitations in this retrospective research. The same number of participants was not possible because of the small number of participants. The participants had different etiologies for dysphagia. As the VFSS data and clinical information were prerecorded, it was inevitable that some data would be missing. Therefore, further investigations need to examine the relation between clinical signs and each physiological event to understand the difference between the aspirators and the non-aspirators with more subjects. In addition to the temporal measures, investigations of biomechanical measurement, including displacement measures (e.g., the maximum length of hyolaryngeal excursion) are needed to examine the ratio or rate of movement of target physiological events. It will help clinicians to identify more useful measures to distinguish aspirators from non-aspirators in pediatric populations.
Despite the limitations, possible management strategies will be suggested based on the findings of this study. First, modification of bolus consistency can be suggested for children who had delayed pharyngeal swallowing and initiation of laryngeal closure. Thickened liquids are thought to increase sensory inputs, move slowly to allow more time for airway closure, and reduce the occurrence of penetration and aspiration ( Fraizer & Friedman, 1996; Garcia, Chambers, & Molander, 2005; Khoshoo, Ross, Kelly, Edell, & Brown, 2001; Mercado-Deane et al., 2001). A chin-tuck position is also a useful technique for children with prolonged DPS and ILC. A chin-tuck widens the valleculae, allowing them to hold the bolus for a certain time. This contributes to increased time for laryngeal closure and decreased aspiration risk ( Larnert & Ekberg, 1995). Several environmental cues, including placing an extra pillow behind the child’s head, positioning the tray low and close to the child’s trunk, and providing verbal cues for the child to look down, can help children perform a chin-tuck during mealtime ( Logemann, 1998; Logemann et al., 2000). Lastly, children with poor bolus transition during the pharyngeal stage (i.e., longer PTT) in this study showed residue after the swallow. For these children, a dry swallow can be used to clear the residue ( Newman, 2000). For children who showed prolonged PTT and ILC as well as reduced laryngeal closure duration (LCD) and duration of UES opening (DUESO), effortful swallow, supraglottic swallow, and Mendelsohn maneuver can be suggested to improve bolus clearance in the pharynx and airway protection. However, these maneuvers should be performed for children who are capable of understanding the procedures of each maneuver ( Morgan, Dodrill, & Ward, 2012).
Figure 1.
Anatomical and physiological references for temporal measurements.
A= initiation of tongue elevation; B= bolus head reaching the valleculae; C= bolus tail passing the UES; D= initiation of laryngeal elevation; E= initial contact of arytenoids and epiglottis; F= final contact of arytenoids and epiglottis; G= initiation of opening of the UES; H= closure of the UES.
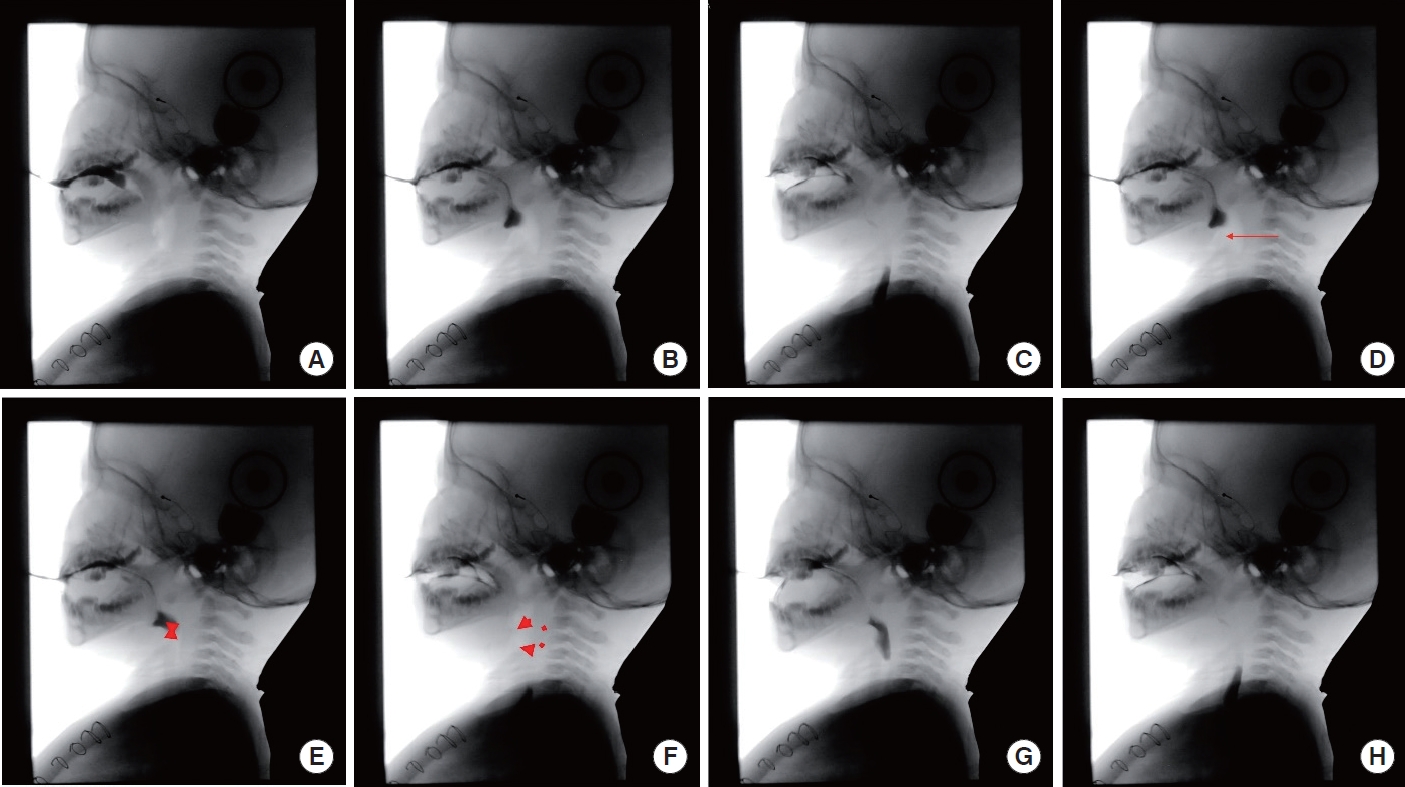
Table 1.
Demographics and clinical information for two groups
|
Information |
Aspirators (N= 16) |
Non-aspirators (N= 26) |
Total (N= 42) |
|
Age (Range) (mo) |
21.2 ± 8.3 (12-42) |
26.3 ± 8.4 (14-46) |
24.7 ± 8.8 (12-46) |
|
Sex |
9 female, 5 male |
14 female, 12 male |
23 female, 19 male |
|
Etiology |
|
|
|
|
Neurological disorders |
14 (88%) |
21 (81%) |
35 (84%) |
|
Cardiopulmonary disorders |
1 (6%) |
1 (4%) |
2 (5%) |
|
Congenital abnormalities |
1 (6%) |
3 (11%) |
3 (9%) |
|
Unspecified |
0 |
1 (4%) |
1 (2%) |
|
Feeding mode |
|
|
|
|
Oral feeding |
2 (12%) |
9 (35%) |
11 (26%) |
|
NG tube |
6 (38%) |
12 (46%) |
18 (43%) |
|
PEG |
1 (6%) |
1 (4%) |
2 (5%) |
|
No report |
7 (44%) |
4 (15%) |
11 (26%) |
|
Pneumonia History |
|
|
|
|
No |
5 (31%) |
18 (70%) |
23 (55%) |
|
Yes |
3 (19%) |
4 (15%) |
7 (17%) |
|
No report |
8 (50%) |
4 (15%) |
12 (28%) |
Table 2.
Temporal measures predicting group membership
|
Measure |
F
|
Odds ratioa
|
90% Confidence Interval for odds ratio
|
p value |
|
Lower |
Upper |
|
OTT |
.952 |
3.070 |
.314 |
30.008 |
.331 |
|
DPS |
5.833 |
12.511 |
1.569 |
99.761 |
.018*
|
|
PTT |
3.987 |
5.782 |
1.011 |
33.072 |
.049*
|
|
ILC |
5.435 |
11.185 |
1.432 |
87.355 |
.022*
|
|
D to LC |
.121 |
.230 |
.000 |
1,011.815 |
.729 |
|
LCD |
.426 |
3.969 |
.030 |
261.183 |
.515 |
|
DUESO |
1.194 |
.035 |
.000 |
15.492 |
.277 |
Table 3.
Combination of delayed pharyngeal swallow (DPS), pharyngeal transit time (PTT), and initiation of laryngeal closure (ILC) predicting group membership
|
Combinations |
F
|
df1 |
df2 |
p value |
|
DPS+PTT+ILC |
2.489 |
3 |
96 |
.065 |
|
DPS+PTT |
3.650 |
2 |
97 |
.030*
|
|
DPS+ILC |
2.929 |
2 |
97 |
.058 |
|
PTT+ILC |
3.529 |
2 |
97 |
.033*
|
REFERENCES
Arvedson, J. C. (2008). Assessment of pediatric dysphagia and feeding disorders: clinical and instrumental approaches. Developmental Disabilities Research Reviews, 14(2), 118–127.   Arvedson, J. C., Rogers, B., Buck, G., Smart, P., & Msall, M. (1994). Silent aspiration prominent in children with dysphagia. International Journal of Pediatric Otorhinolaryngology, 28(2-3), 173–181.   Dodrill, P., & Gosa, M. M. (2015). Pediatric dysphagia: physiology, assessment, and management. Annals of Nutrition & Metabolism, 66(5), 24–31.   Erasmus, C. E., van Hulst, K., Rotteveel, J. J., Willemsen, M. A. A. P., & Jongerius, P. H. (2012). Clinical practice. European Journal of Pediatrics, 171(3), 409–414.    Fraizer, J. B., & Friedman, B. (1996). Swallow function in children with Down syndrome: a retrospective study. Developmental Medicine & Child Neurology, 38(8), 695–703.   Friedman, B., & Frazier, J. B. (2000). Deep laryngeal penetration as a predictor of aspiration. Dysphagia, 15(3), 153–158.  Garcia, J. M., Chambers IV, E., & Molander, M. (2005). Thickened liquids. American Journal of Speech-Language Pathology, 14(1), 4–13.   Han, T., Bang, M., Chung, S., Shin, H., & Jeon, J. (2001). The pattern of malnutrition in cerebral palsy and relating factors. Journal of Korean Academy of Rehabilitation Medicine, 25(1), 18–25.
Han, Y., Kim, Y., Yi, Y., & Oh, B. (2024). Temporal characteristics of oropharyngeal swallowing in young children with dysphagia. Journal of the Korean Dysphagia Society, 14(1), 31–40.  Henderson, M., Miles, A., Holgate, V., Peryman, S., & Allen, J. (2016). Application and verification of quantitative objective videofluoroscopic swallowing measures in a pediatric population with dysphagia. Journal of Pediatrics, 178, 200–205.   Kahrilas, P. J., Lin, S., Rademaker, A. W., & Logemann, J. A. (1997). Impaired deglutitive airway protection: a videofluoroscopic analysis of severity and mechanism. Gastroenterology, 113(5), 1457–1464.   Kendall, K. A., & Leonard, R. J. (2001). Bolus transit and airway protection coordination in older dysphagic patients. The Laryngoscope, 111(11), 2017–2021.  Khoshoo, V., Ross, G., Kelly, B., Edell, D., & Brown, S. (2001). Benefits of thickened feeds in previously healthy infants with respiratory syncytial viral bronchiolitis. Pediatric Pulmonology, 31(4), 301–302.  Larnert, G., & Ekberg, O. (1995). Positioning improved the oral and pharyngeal swallowing function in children with cerebral palsy. Acta Paediatrica, 84(6), 689–692.   Lefton-Grief, M. A. (2008). Pediatric dysphagia. Physical Medicine & Rehabilitation Clinics of North America, 19(4), 837–851.   Lefton-Grief, M. A., & Arvedson, J. C. (2008). Schoolchildren with dysphagia associated with medically complex conditions. Language Speech & Hearing Services in Schools, 39(2), 237–248.  Leonard, R. J., Kendall, K. A., McKenzie, S., Gonçalves, M. I., & Walker, A. (2000). Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia, 15(3), 146–152.   Logemann, J. A. (1998). Evaluation and treatment of swallowing disorders (2nd ed.) Austin: PRO-ED, Inc.
Logemann, J. A., Rademaker, A. W., Kahrilas, P. J., & Smith, C. H. (2000). Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of Speech, Language, & Hearing Research, 43(5), 1264–1274.   Lustre, N. D. S., Freire, T. R. B., & Silverio, C. C. (2013). Temporal measurements of oral transit time in children with cerebral palsy of different levels motors and the relationship with the severity of dysphagia. Audiology-Communication Research, 18(3), 155–161.
Martin-Harris, B., Carson, K. A., Pinto, J. M., & Lefton-Greif, M. A. (2019). BaByVFSSImP© a novel measurement tool for videofluoroscopic assessment of swallowing impairment in bottle-fed babies: establishing a standard. Dysphagia, 35, 90–98.    Mercado-Deane, M., Burton, E. M., Harlow, S. A., Glover, A. S., Deane, D. A., Guill, M. F., & Hudson, V. (2001). Swallowing dysfunction in infants less than 1 year of age. Pediatric Radiology, 31(6), 423–428.   Morgan, A. T., Dodrill, P., & Ward, E. C. (2012). Interventions for oropharyngeal dysphagia in children with neurological impairment. Cochrane Database of Systematic Reviews, 2012(10), 1–37.   Newman, L. A. (2000). Optimal care patterns in pediatric patients with dysphagia. Seminars in Speech & Language, 21(4), 281–291.   Newman, L. A., Cleveland, R. H., Blickman, J. G., Hillman, R. E., & Jaramillo, D. (1991). Videofluoroscopic analysis of the infant swallow. Investigative Radiology, 10, 870–873.   Newman, L. A., Keckley, C., Petersen, M. C., & Hamner, A. (2001). Swallowing function and medical diagnoses in infants suspected of dysphagia. Pediatrics, 108(6), 1–4.   Park, T., Kim, Y., Ko, D., & McCullough, G. H. (2010). Initiation and duration of laryngeal closure during the pharyngeal swallow in post-stroke patients. Dysphagia, 25(3), 177–182.   Perlman, A. L., Booth, B. M., & Grayhack, J. P. (1994). Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia, 9(2), 90–95.   Power, M. L., Hamdy, S., Goulermas, J. Y., Tyrrell, P. J., Turnbull, I., & Thompson, D. G. (2009). Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia, 24(3), 257–264.   Robbins, J., Levine, R. L., Maser, A., Rosenbek, J. C., & Kempster, G. B. (1993). Swallowing after unilateral stroke of the cerebral cortex. Archives of Physical Medicine & Rehabilitation, 74(12), 1295–1300.   Roden, D. F., & Altman, K. W. (2013). Causes of dysphagia among different age groups. Otolaryngologic Clinic of North America, 46(6), 965–987.   Steele, C. M., & Cichero, J. A. Y. (2014). Physiological factors related to aspiration risk: a systematic review. Dysphagia, 29(3), 295–304.    Stevenson, R. D., & Allaire, J. H. (1991). The development of normal feeding and swallowing. Pediatric Clinics of North America, 38(6), 1439–1453.   Tutor, J. D., & Gosa, M. M. (2012). Dysphagia and aspiration in children. Pediatric Pulmonology, 47(4), 321–337.   Velayutham, P., Irace, A. L., Kawai, K., Dodrill, P., Perez, J., Londahl, M., ..., & Rahbar, R. (2018). Silent aspiration: who is at risk. The Laryngoscope, 128(8), 1952–1957.   Weckmueller, J., Easterling, C., & Arvedson, J. (2011). Preliminary temporal measurement analysis of normal oropharyngeal swallowing in infants and young children. Dysphagia, 26(2), 135–143.   Weir, K., McMahon, S., Barry, L., Ware, R., Masters, I. B., & Chang, A. B. (2007). Oropharyngeal aspiration and pneumonia in children. Pediatric Pulmonology, 42, 1024–1031.   Yip, H., Leonard, R., & Belafsky, P. C. (2006). Can a fluoroscopic estimation of pharyngeal constriction predict aspiration. Otolaryngology Head & Neck Surgery, 135(2), 215–217.  
|
|






